Nature of Acids and Bases
Nature of Acids and Bases: Overview
This topic covers concepts, such as, Common Element Present in All Acids, Conduction of Electricity in Acidic Solutions, Dilution of a Base with Water & Alkalies etc.
Important Questions on Nature of Acids and Bases
A solution of a base with pH is given.
Which of the following can be done to decrease its pH?
P) add distilled water to it.
Q) add a solution of a different base with pH 8.7.
R) add a few drops of an acid with an unknown pH.
What is the meaning of dilution of acids and bases?
gas can be dried by passing it over:
In which of the following oxalic acid is found naturally
The bulb is not glowing in this experimental setup. Because:
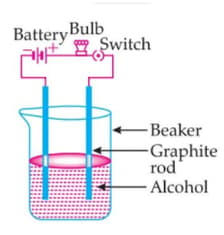
The aqueous solution of hydrogen chloride contains
Which of the following ionisation equation for acid or base is wrong?
As a result of the interaction of these oxides with water. Compounds X, Y, Z, and T are formed. Which of these compounds is named incorrectly?
Which of the following can act as an antacid?
If a base dissolves in water by what name is it better known?
Which one is the strongest acid amongst these -
Identify the hydronium ion among the following.
What happens when a base is mixed with water?
_____ act as an antacid to cure acidity.
